Serration pattern analysis as a practical adjunct tool forcategorization of subepidermal autoimmune blistering diseases
Sukriti Arora, Varsha M. Shetty, Chythra R. Rao1, Satish B. Pai, Raghavendra Rao
Departments of Dermatology, 1Community Medicine, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, India
Abstract
- Background: Serration pattern analysis helps in the classification of subepidermal autoimmune blistering disorders; more precisely, it helps to differentiate epidermolysis bullosa acquisita from other subepidermal autoimmune blistering disorders. Most of the published reports of this tool have come from a single center.
- Objectives: The objectives of the study were to study the utility of serration pattern analysis in classifying subepidermal autoimmune blistering disorders
- Methods: Seventy five cases of subepidermal autoimmune blistering disorders were enrolled in this prospective study. Athree millimeter punch biopsy was taken from the perilesional skin or mucosa for direct immunofluorescence; indirect immunofluorescence was carried out using salt-split skin. Subclassification of subepidermal autoimmune blistering disorders was done based on direct immunofluorescence, indirect immunofluorescence on salt-split skin, indirect immunofluorescence using knockout skin and serration pattern analysis findings.
- Results: Indirect immunofluorescence was positive in 68 cases; 14 cases showed a dermal staining pattern while the rest showed either an epidermal or a combined pattern. All patients with epidermal or combined staining patterns showed “n” serrated pattern on direct immunofluorescence. Nine patients with dermal staining on indirect immunofluorescence also revealed an “n” serration pattern on direct immunofluorescence indicating the diagnosis of anti-p200 pemphigoid, and the rest showed a “u” serrated pattern. Three patients with negative indirect immunofluorescence showed “u” serration on direct immunofluorescence while the rest showed “n” serration.
- Limitations: ELISA and immunoblotting could not be performed due to resource constraints.
- Conclusion: Based on indirect immunofluorescence and serration pattern analysis, classification of the majority of patients with subepidermal autoimmune blistering disorders was possible in our study. Pattern recognition is a cost-effective tool and can be easily learnt. It is recommended to be practiced in all laboratories where facilities for advanced immunological diagnosis are unavailable.
- Key words: Serration pattern, immunofluorescence, subepidermal autoimmune blistering disorders
Plain Language Summary
Subepidermal autoimmune blistering diseases are a heterogenous group of diseases characterised clinically by the presence of tense blisters with or without painful erosions in the mouth. It occurs because of the altered defence mechanism - body’s defence cells which are primed to protect us, instead produces substances known as autoantibodies against the proteins present at the junction between the top layer (the ‘epidermis’) and the bottom layer (the ‘dermis’) of the skin. These proteins play an important role in maintaining the integrity of the skin. In the diseased skin, there is separation of epidermis from the dermis, resulting in a blister formation. There are at least seven well characterised clinical conditions that exhibit an overlapping feature; hence, clinicians may find it difficult to subcategorise these conditions precisely. It is very important to distinguish them both from therapeutic and prognostic point of view. There are few laboratory test that will come to the rescue of clinicians; some of these tests such as immunoblotting requires expertise and are not available in many places across the globe. In this study we describe a simple technique known as serrated pattern analysis. Though this technique was described in 2004 by a Dutch group, not many centres are practicing it, probably due to the presumed hurdles in the technicalities. In this report, we describe a modification of this procedure. We found this technique very useful in classifying these conditions.
How to cite this article: Arora S, Shetty VM, Rao CR, Pai SB, Rao R. Serration pattern analysis as a practical adjunct tool for categorization of subepidermal autoimmune blistering diseases. Indian J Dermatol Venereol Leprol 2021;87:778-86.
Introduction
Subepidermal autoimmune blistering disorders are a heterogeneous group of diseases characterized immunopathologically by production of autoantibodies against structural components of the dermoepidermal junction.1,2 Seven well-characterized subepidermal autoimmune blistering disorders encountered in clinical practice are bullous pemphigoid, epidermolysis bullosa acquisita, linear IgA dermatosis, mucous membrane pemphigoid, pemphigoid gestationis, lichen planus pemphigoides and anti-p200 pemphigoid. The clinical presentation of subepidermal autoimmune blistering disorders is polymorphic and these various disorders are often difficult to distinguish on clinical grounds alone.3 A precise diagnosis is important as it has therapeutic implications. Certain subtypes of subepidermal autoimmune blistering disorders (e.g. bullous pemphigoid) are amenable to topical steroids alone, low-dose oral corticosteroids or corticosteroid-sparing agents such as dapsone.4 On the other hand, treatment of a few other subtypes such as epidermolysis bullosa acquisita is quite challenging and often requires high doses of prednisolone to control the disease.5 Hence, it is imperative to arrive at an accurate diagnosis before instituting therapy.
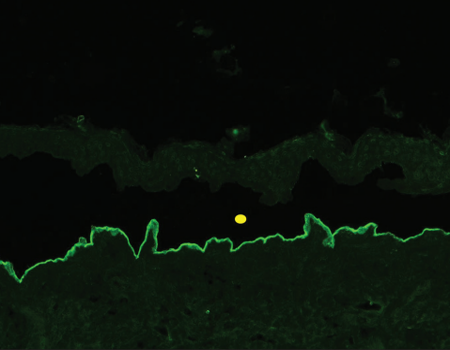
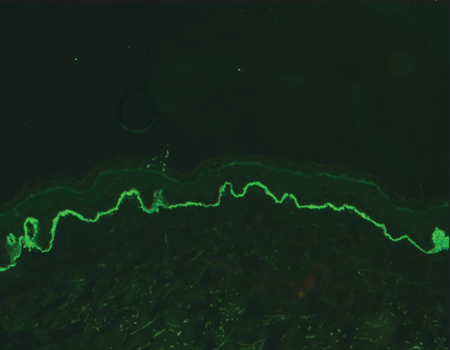
In 2004, Vodegel et al. in Groningen described a unique method of classifying these conditions based on direct immunofluorescence microscopy.6 They observed that epidermolysis bullosa acquisita patients showed a “u” serrated pattern (immunoglobulin deposition between the rootlets of basal keratinocytes giving a “grass-like” appearance), while all other subgroups of patients revealed “n” serrated pattern (immune deposits along the rootlets of the basal keratinocytes) [Figure 1]. In subsequent years, they substantiated these findings and reported that this test could be employed in many other subepidermal autoimmune blistering disorders including mucous membrane pemphigoid.7-10 Recently, another group in Central Europe reported the usefulness of this technique in distinguishing epidermolysis bullosa acquisita from other pemphigoid diseases.Apart from the work reported from these two laboratories in Europe, there is no literature about this technique from other parts of the world. In this study, we aimed to determine the utility of serration pattern analysis for classification of patients with subepidermal autoimmune blistering disorders in our tertiary care dermatology unit.
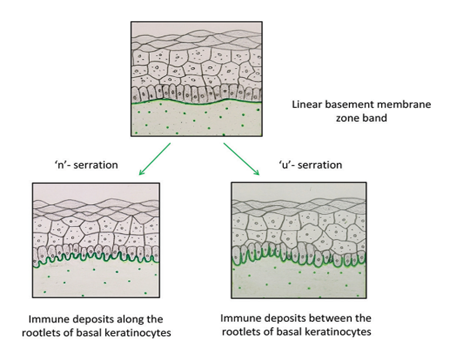
In 2004, Vodegel et al. in Groningen described a unique method of classifying these conditions based on direct immunofluorescence microscopy.6 They observed that epidermolysis bullosa acquisita patients showed a “u” serrated pattern (immunoglobulin deposition between the rootlets of basal keratinocytes giving a “grass-like” appearance), while all other subgroups of patients revealed “n” serrated pattern (immune deposits along the rootlets of the basal keratinocytes) [Figure 1]. In subsequent years, they substantiated these findings and reported that this test could be employed in many other subepidermal autoimmune blistering disorders including mucous membrane pemphigoid.7-10 Recently, another group in Central Europe reported the usefulness of this technique in distinguishing epidermolysis bullosa acquisita from other pemphigoid diseases. Apart from the work reported from these two laboratories in Europe, there is no literature about this technique from other parts of the world. In this study, we aimed to determine the utility of serration pattern analysis for classification of patients with subepidermal autoimmune blistering disorders in our tertiary care dermatology unit.
Patients and Methods
-
Patients
A total of 75 patients visiting the Kasturba Medical College and Hospital, Manipal, were enrolled prospectively over a period of 15 months. Subjects were included if they fulfilled the following criteria:- had a provisional clinical diagnosis of subepidermal autoimmune blistering disorders,
- consented to undergo a perilesional biopsy for direct immunofluorescence and
- consented to a blood draw for indirect immunofluorescence. Patients provisionally diagnosed with subepidermal autoimmune blistering disorders but who underwent only one of the tests (direct immunofluorescence or indirect immunofluorescence) were excluded from the study. Patient demographics and clinical information were recorded in a predesigned proforma. The Institutional Ethical Committee approved the study (IEC/583/2017) and written informed consent was obtained from all the study participants.
- Sample collection
A three millimeter punch biopsy was obtained from the perilesional skin (n = 70) or mucosa (n = 5) under local infiltrative anesthesia (lignocaine 2% with adrenaline). Fifty six specimens were sent to the laboratory in normal saline. Biopsies that could not be transported to the laboratory within 24 h were stored in Michel’s medium (n = 19). In addition, five milliliters of blood were obtained from each participant in plain Vacutainer and the serum separated. Serum was stored in a deep freezer at −80°C until further use. - Direct immunofluorescence
The biopsy specimens were washed in phosphate-buffered saline in a rotator at 4°C in a refrigerator for 12 h. The specimens were then oriented and embedded in optimal cutting temperature compound (Cryomatrix, Thermo Fisher Scientific, Waltham, USA). The blocks were next snap frozen in liquid nitrogen.
Frozen sections of six micrometer thickness were taken on polytetrafluoroethylene (PTFE)coated glass slides (Hendley, Essex, UK); the polytetrafluoroethylene coating acts as a liquid blocker and prevents cross-contamination of wells. The slides were washed with phosphate-buffered saline for ten minutes and fan dried. The frozen sections were then stained with fluorescein isothiocyanate labeled antibodies with specificities against IgG, IgM, IgA, C3 and fibrinogen (DAKO, Glostrup) at appropriate dilution as per standard department protocol (IgG and fibrinogen in 1:400 dilution while the others with 1:200 dilution). The slides were then incubated at room temperature in a moist chamber. At the end of 60 min, the slides were washed with phosphatebuffered saline for 30 min (three washes of ten minutes each) and fan dried. These were then mounted in buffered glycerol and examined under a fluorescence microscope (Zeiss Axioimager, Oberkochen). The slides were read at ×10, ×20, ×40 and ×100 (oil immersion) objectives. - Observers
The two observers (RR and SA) were blinded to the clinical diagnosis and reported the serration pattern independently at two different sittings to eliminate bias. Both the observers enrolled themselves for the online course on serration pattern at http://www.nversusu.umcg.nl/and made themselves familiar with the pattern analysis. - Indirect immunofluorescence on salt-split skin
Normal human skin was incubated overnight with one molar sodium chloride at room temperature. The normal human skin was then gently teased with a blunt forceps to separate the epidermis from dermis at the level of the lamina lucida. Subsequently, six micrometers frozen section of this substrate was obtained as described above and incubated with the patient’s serum (diluted 1:10 in phosphate-buffered saline) for one hour in a moist chamber at room temperature. The sections were washed with phosphate-buffered saline for ten minutes, fan dried and incubated with fluorescein isothiocyanate labeled anti-human IgG and IgA antibodies (diluted 1:100 in phosphate-buffered saline) for one hour in a moist chamber. The sections were then washed in phosphatebuffered saline, fan dried, mounted in buffered glycerol and examined under a fluorescence microscope. - Indirect immunofluorescence using knock-out skin
Sera that showed a dermal staining pattern on indirect immunofluorescence-salt-split skin were further studied on hereditary epidermolysis bullosa skin lacking Type VII collagen (recessive dystrophic epidermolysis bullosa/RDEB) and laminin 332 (severe, generalized junctional epidermolysis bullosa) by a modified indirect immunofluorescence technique as described previously.12 - Data analysis
Data were entered and analyzed using the Statistical Package for the Social Sciences (Statistical Package for the Social Sciences Inc. Released 2006. Statistical Package for the Social Sciences for Windows, Version 15.0. Chicago). Quantitative data have been summarized as percentages and proportions. Descriptive statistics were used; mean ± standard deviation was used as a summary statistic for continuous variables. Chi-square test was employed for univariate analysis of categorical variables and Fisher’s exact test was reported when appropriate. All tests were two tailed and P < 0.05 was considered to be statistically significant.
Results
Patient demographics and clinical information
The age of the patients ranged from five to 84 years (mean age, 55.75 ± 17.69 years). Thirty two (42.7%) patients wereabove the age of 60 years. There were 42 females (56%) and 33 males (44%). Based on history and clinical examination, a provisional diagnosis of bullous pemphigoid was made in 46 (61.3%) patients. Clinical diagnosis of mucous membrane pemphigoid (five patients), epidermolysis bullosa acquisita (five patients), lichen planus pemphigoides (four patients) and linear IgA dermatosis (two patients) were made in 16
others. A precise clinical diagnosis could not be made in
13 (17.3 %) patients and a differential diagnosis of various
subepidermal autoimmune blistering disorders (bullous
pemphigoid, epidermolysis bullosa acquisita or linear IgA
dermatosis) was considered for them. Four patients of lichen
planus pemphigoides showed typical lesions of lichen planus;
one patient with bullous pemphigoid had associated psoriasis.
Direct immunofluorescence
Direct immunofluorescence microscopy revealed linear staining
of the basement membrane zone (BMZ) in all 75 patients. Fifty
three biopsy specimens revealed a combination of IgG and C3;
12 specimens showed basement membrane zone deposition
with three or more immunoreactants (IgG, C3, IgA, IgM or
fibrinogen). The basement membrane zone stained with a
single immunoreactant in ten specimens (seven showed either
IgG or C3 staining alone and three showed IgA alone).
Indirect immunofluorescence
Circulating autoantibodies were detected by the indirect
immunofluorescence-salt-split skin technique in 68 patients
(91.3%). An epidermal (“roof”) staining pattern was detected in
49 patients (65.3%) while a combined (epidermal and dermal)
pattern was observed in five patients (6.7%). Fourteen patients
(18.7%) showed an exclusive dermal (“floor”) staining pattern.
Serration pattern analysis
Using direct immunofluorescence microscopy and a ×100
objective under oil immersion, we could discern either “n” or
“u” serrated patterns in all 75 specimens. Sixty seven patients
showed an “n” serrated pattern while eight patients showed
a “u” serrated pattern. The relationship between indirect
immunofluorescence results and serration pattern is depicted
in Table 1. All patients who showed epidermal or combined
patterns (n = 54) on indirect immunofluorescence revealed the
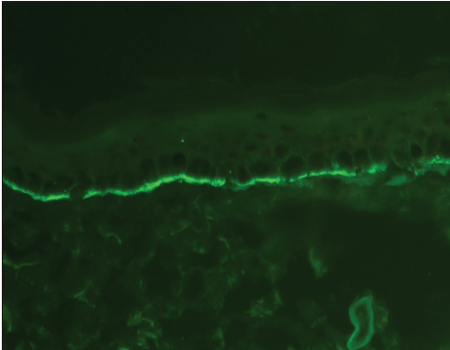
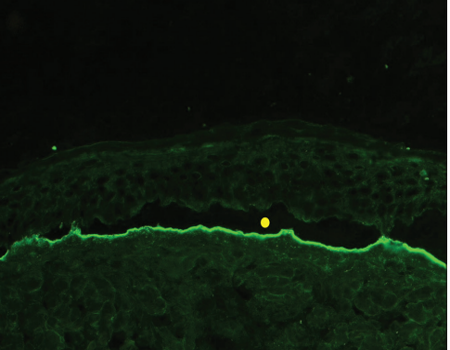
“n” serrated pattern on direct immunofluorescence [Figure 2].
Nine out of the 14patients (64.3%) who showed dermal staining
on indirect immunofluorescence microscopy also revealed an “n” serrated pattern while the remaining five patients showed
a “u” serrated pattern [Table 2]. Indirect immunofluorescence
was negative in seven patients [Table 3]; three of these
showed the “u” serrated pattern while the remaining patients
showed “n” serration on direct immunofluorescence. The
association between the indirect immunofluorescence pattern
and the serration pattern with direct immunofluorescence for
subepidermal autoimmune blistering disorders was found to
be statistically significant (P < 0.01) by Fisher’s exact test
with 100% sensitivity of the “n” serrated pattern for roof
staining on indirect immunofluorescence.
We did not observe any differences between biopsy specimens
transported in normal saline and Michel’s medium with
respect to serrated pattern recognition.
| Table 1: Relationship between indirect immunofluorescence pattern and serration pattern analysis for subepidermal autoimmune blistering disorders (n=75) | |||||
|---|---|---|---|---|---|
| Serration pattern* | Indirect immunofluorescence | Total | |||
| Roof n (%) | Roof and Floor n (%) | Floor n (%) | Negative n (%) | ||
| “n” serrated | 49(100) | 5(100) | 9(64.3) | 4(57.1) | 67 |
| “u” serrated | 0 | 0 | 5(35.7) | 3(42.9) | 8 |
| Total | 49 | 5 | 14 | 7 | 75 |
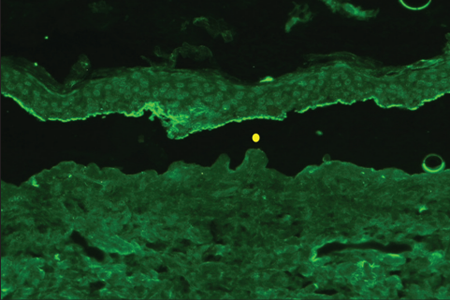
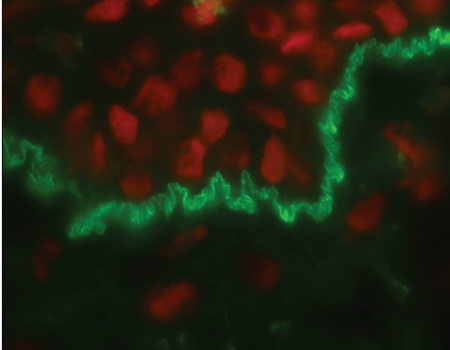
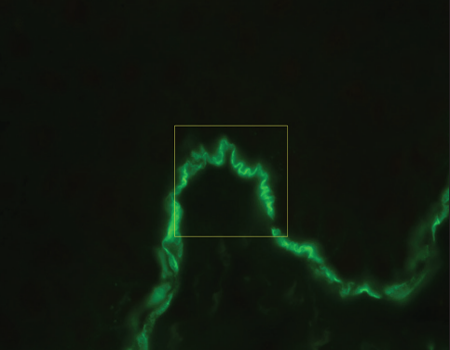
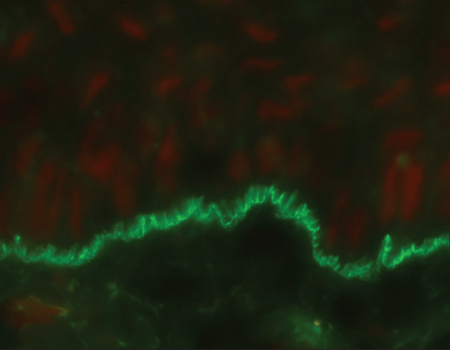
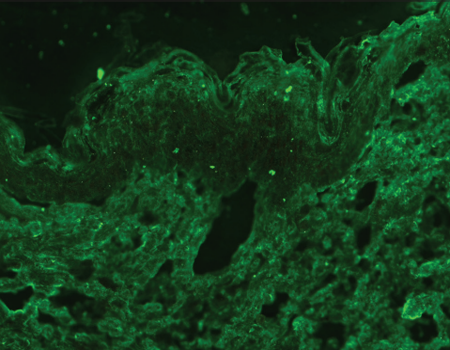
Indirect immunofluorescence using knock-out skin
Indirect immunofluorescence using knock-out epidermolysis
bullosa skin was carried out with the sera of 14 patients who
demonstrated dermal staining [Figures 3 and 4]. Cases which
showed basement membrane zone staining with laminin 332
knock-out skin but not in the Type VII collagen-deficient skin
were labeled as epidermolysis bullosa acquisita. Basement
membrane zone staining in recessive dystrophic epidermolysis
bullosa skin was absent in five sera; it was positive in all
14 patients in skin deficient in laminin 332 [Table 2], thus
confirming the diagnosis of epidermolysis bullosa acquisita.
Clinicoimmunopathological correlation
Using a combination of direct immunofluorescence,
indirect immunofluorescence on salt-split skin and indirect
immunofluorescence on knock-out epidermolysis bullosa skin,
we could precisely subcategorize 73 out of 75 subepidermal
autoimmune blistering disorders in our patients. Table 4
shows the final diagnosis based on the laboratory parameters.
The concordance between the clinical and final diagnosis in
patients with bullous pemphigoid was 82.6% (38/46). In the
remaining eight patients, a diagnosis of anti-p200 pemphigoid
was made in six patients (dermal staining on indirect
immunofluorescence and “n” serrated pattern on direct
immunofluorescence) and epidermolysis bullosa acquisita
in two patients (dermal staining pattern and “u” serrated
pattern on direct immunofluorescence). A concordance
rate of 100% was observed among patients with mucous
membrane pemphigoid and lichen planus pemphigoides
(“n” serration on direct immunofluorescence and a positive
| Table 2: Clinical and immunological characteristics of patients with a “floor” staining pattern on indirect immunofluorescence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. No. | Age | Sex | Generalized vesicles and bullae | Acral lesions | Mucosal involvement | Phenotype | DIF | Serration pattern | BMZ staining in Type VII collagendeficient skin | BMZ staining in Lam 332 deficient skin | Final diagnosis |
| 1. | 14 | F | Yes | Yes | Yes | Mechanobullous | IgG,C3 | u | Absent | Present | EBA |
| 2. | 49 | M | Yes | Yes | Yes | Mechanobullous | IgG,C3 | u | Absent | Present | EBA |
| 3. | 12 | F | Yes | Yes | No | Inflammatory | IgG, C3 | u | Absent | Present | EBA |
| 4. | 52 | M | Yes | No | No | Inflammatory | IgG, C3 | u | Absent | Present | EBA |
| 5 | 5 | M | Yes | No | Yes | Inflammatory | IgG, C3 | n | present | Present | Anti-p-200 pemphigoid |
| 6 | 75 | M | Yes | No | Yes | Inflammatory | IgG, C3 | n | Present | Present | Anti-p-200 pemphigoid |
| 7. | 65 | F | Yes | No | No | Inflammatory | IgG, C3,IgA | n | Present | Present | Anti-p-200 pemphigoid |
| 8 | 62 | F | Yes | No | Yes | Mechanobullous | IgG, C3 | u | Absent | Present | EBA |
| 9 | 58 | M | Yes | No | Yes | Inflammatory | IgG, C3 | n | Present | Present | Anti-p-200 pemphigoid |
| 10 | 56 | M | No | Yes | Yes | Inflammatory | IgG, C3, IgA | n | Present | Present | Anti-p-200 pemphigoid |
| 11 | 60 | F | Yes | No | No | Inflammatory | C3, IgA | n | Present | Present | Anti-p-200 pemphigoid |
| 12 | 55 | F | Y | No | Yes | Inflammatory | IgG, C3 | n | Present | Present | Anti-p-200 pemphigoid |
| 13. | 84 | F | Y | No | No | Inflammatory | C3 | n | Present | Present | Anti-p-200 pemphigoid |
| 14 | 28 | F | Y | No | No | Inflammatory | IgG, C3 | n | Present | Present | Anti-p-200 pemphigoid |
| Table 3: Clinical and immunological characteristics of patients with negative indirect immunofluorescence (n=7) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. No. | Age | Sex | Distribution | Acral lesions | Mucosal involvement | Clinical diagnosis | DIF | Serration pattern analysis | Final diagnosis | ||
| 1. | 47 | Female | Localized | No | No | Subepidermal autoimmune blistering disorders | IgG, C3 | n | Bullous pemphigoid/anti-p200 pemphigoid | ||
| 2. | 67 | Male | Oral | No | Yes | Mucous membrane pemphigoid | IgG, C3 | n | Mucous membrane pemphigoid | ||
| 3. | 61 | Female | Generalized | No | No | Epidermolysis bullosa acquisita | IgG, C3, IgA, IgM | n | Bullous pemphigoid/anti-p200 pemphigoid | ||
| 4. | 61 | Female | Localized | No | No | Subepidermal autoimmune blistering disorders | IgA | u | IgA epidermolysis bullosa acquisita | ||
| 5 | 13 | Male | Generalized | No | Yes | Subepidermal autoimmune blistering disorders | IgG, C3,IgA | u | Epidermolysis bullosa acquisita | ||
| 6 | 51 | Male | Generalized | No | Yes | Lichen planus pemphigoides | IgG, C3 | n | Lichen planus pemphigoides | ||
| 7. | 20 | Female | Generalized | No | No | Bullous pemphigoid | IgG, C3,IgA | u | Epidermolysis bullosa acquisita | ||
epidermal staining on indirect immunofluorescence). On the other hand, concordance between clinical and immunological diagnosis was 40% (2/5) in epidermolysis bullosa acquisita. A revised diagnosis of bullous pemphigoid was made in two patients (epidermal staining on indirect immunofluorescence and “n” serration on direct immunofluorescence) and anti-p200 pemphigoid in one patient (dermal staining on indirect immunofluorescence but “n” serration on direct immunofluorescence). Among 13 patients who had more than one possible clinical diagnosis, immunological tests (direct immunofluorescence, indirect immunofluorescence, indirect immunofluorescence on knock-out skin and serration pattern analysis) confirmed the diagnosis of bullous pemphigoid in six, epidermolysis bullosa acquisita in three, anti-p200 pemphigoid in two and linear IgA dermatosis in one patient. One patient with a clinical diagnosis of subepidermal autoimmune bullous disease was tested negative with indirect immunofluorescence but showed “n” serrated pattern on direct immunofluorescence; hence, we could not distinguish bullous pemphigoid from anti-p200 pemphigoid in this patient.
Circulating antibodies could not be detected in seven
patients. Three of them showed a “u” serrated pattern on
direct immunofluorescence, thus indicating the diagnosis of
epidermolysis bullosa acquisita. One among these showed linear
IgA staining on direct immunofluorescence microscopy and “u”
serration, pointing to a diagnosis of IgA epidermolysis bullosa
acquisita. Among patients in this group who showed “n” serrated
pattern (n = 4), one patient each was suffering from lichen planus
pemphigoides and mucous membrane pemphigoid. Although the
possibility of epidermolysis bullosa acquisita was ruled out in the
two other patients with negative indirect immunofluorescence,
we could not distinguish anti-p200 pemphigoid from bullous
pemphigoid as both patients revealed “n” serrated pattern.
Thus, overall bullous pemphigoid emerged as the commonest
subepidermal autoimmune blistering disorder in this study
with 45 patients followed by anti-p200 pemphigoid (nine
patients) and epidermolysis bullosa acquisita (eight patients).
The average age of patients with bullous pemphigoid was
60 years while that of those with anti-p200 pemphigoid was
54 years and epidermolysis bullosa acquisita was 35.4 years.
None of the patients had been suspected to have anti-p200
pemphigoid clinically [Table 4]. Seven patients with anti-p200
pemphigoid had generalized lesions; mucosal lesions were
detected in five. However, only one patient had acral lesions
involving palms and soles. None had associated psoriasis.
Discussion
Direct immunofluorescence microscopy remains the gold standard test to demonstrate autoantibodies that are bound to antigens at the dermoepidermal junction in patients with subepidermal autoimmune blistering disorders. It characteristically shows the presence of linear IgG, C3 or IgA deposition at the level of the basement membrane zone. Although direct immunofluorescence findings confirm the diagnosis of subepidermal autoimmune blistering disorders, it cannot be used as a standalone test for their subclassification.3 In 1984, Gammon et al. described a simple and reliable indirect immunofluorescence technique using the patient’s serum and artificially split skin as a substrate; they subcategorized subepidermal autoimmune blistering disorders into “epidermal binding” and “dermal binding” groups.13 While epidermal (“roof”) staining is highly specific for bullous pemphigoid, dermal staining is not specific for any single disease.
| Table 4: Correlation between initial clinical diagnosis with revised diagnosis after immunological tests (n=75) | |||||||
|---|---|---|---|---|---|---|---|
| Clinical diagnosis BP | IRevised diagnosis | ||||||
| BP | EBA | LAD | LPP | MMP | P-200 | BP/P200 | |
| BP (46) | 38 | 2 | 0 | 0 | 0 | 6 | 0 |
| EBA (5) | 1 | 2 | 0 | 0 | 0 | 1 | 1 |
| LAD (2) | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| LPP (4) | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Subepidermal autoimmune blistering disorders (13) | 6 | 3 | 1 | 0 | 0 | 2 | 1 |
| Total | 45 | 8 | 2 | 4 | 5 | 9 | 2 |
It can be seen in epidermolysis bullosa acquisita, anti-p200
pemphigoid and anti-laminin 332 type of mucous membrane
pemphigoid.15,16 Another diagnostic pitfall in the latter subgroup
is that circulating autoantibodies may not always be detectable
by indirect immunofluorescence. The previous studies have
reported a negative indirect immunofluorescence in about
53% of epidermolysis bullosa acquisita patients and 20–50%
of patients with mucous membrane pemphigoid.10,17,18 Hence,
additional investigations such as ELISA, immunoblotting and
immunoprecipitation may be required; however, these tests
are performed only in certain research laboratories around the
world and may not be accessible to the treating physician.8
Serration pattern analysis of direct immunofluorescence on
biopsy samples may be a useful tool in such circumstances as
it can be performed in any diagnostic laboratory which has a
facility for direct immunofluorescence microscopy
In this study, we could identify the serration pattern on direct
immunofluorescence microscopy in all 75 patients. Even though
this technique was described more than a decade ago, it has not
found a place in routine diagnostic algorithms across the world,
perhaps due to presumed technical hurdles. Vodegel et al. and
Terra et al. reported the need for thinner frozen sections (four
micrometers) and higher magnification of ×40 to obtain the
optimum result.6,9 Meijer et al. in a recent report documented that
serration can be appreciated even in six micrometers sections
using a ×60 objective.11 We too could visualize the serration
pattern in six micrometers sections; however, serration could
not be clearly demonstrated using a ×40 objective. Since our
microscope was not equipped with a ×60 objective, we used the
×100 objective. Software to enhance the image quality reported
in a previous study was not utilized in the current study.
Together bullous pemphigoid, anti-p200 pemphigoid and
epidermolysis bullosa acquisita accounted for 82.7% of the
subepidermal autoimmune blistering disorders in the present
study. Bullous pemphigoid characteristically shows an
epidermal staining pattern on indirect immunofluorescence
while the latter two diseases show a dermal staining pattern.
Anti-p200 pemphigoid was diagnosed in 64.3% of the cases
with “dermal binding” subepidermal autoimmune blistering
disorders in the present study. This is in line with Lau et al.
who reported that 78.7% of dermal binding subepidermal
autoimmune blistering disorders were anti-p200 pemphigoid.
Diagnosis of anti-p200 pemphigoid was made in our study by
excluding epidermolysis bullosa acquisita only. Immunoblotting
might have confirmed the presence of antibodies against the
p-200 protein in these patients; alternatively, it might have shown
reactivity against other cross-reacting proteins at the basement
membrane zone. Due to resource constraints, the study results
could not be correlated with advanced immunological tests
such as ELISA and immunoblotting, and this is a limitation in
our study. Although immunoblotting is required for the final
diagnosis of anti-p200 pemphigoid, this technique is currently
available only in a few research laboratories. In such a scenario,
a combination of indirect immunofluorescence and serration
pattern analysis may be a useful alternative.
Anti-p200 pemphigoid is clinically indistinguishable from
bullous pemphigoid, linear IgA dermatosis and the inflammatory
variant of epidermolysis bullosa acqusita.20,21 It affects a
relatively younger population compared to bullous pemphigoid
which concurs with the present study findings.22 Psoriasis
has been reported to coexist in about one-third of anti-p200
pemphigoid cases in a Japanese study; however, this finding
has not been substantiated in Caucasians.15,22 Acral blistering
resembling dyshidrosiform lesions are highly suggestive of
anti-p200 pemphigoid; however, it was observed only in
one patient in the present study. Although not a predominant
feature, mucosal involvement may be found in 20–50% of
these patients.14,20 Oral mucosal involvement was seen in 55.5%
(five out of nine) of patients in the present study. None of our
patients showed signs of scarring or milia formation. In general,
anti-p200 pemphigoid has a benign course compared to bullous
pemphigoid and epidermolysis bullosa acquisita.
Two major types of presentation of epidermolysis bullosa
acquisita are mechanobullous and inflammatory form
(resembling bullous pemphigoid or other subepidermal
autoimmune blistering disorders); the latter accounts for
two-thirds of epidermolysis bullosa acquisita cases.7,22 A
majority of the cases (n = 5) in the present study had the
inflammatory phenotype. Autoantibodies in epidermolysis
bullosa acquisita are directed against Type VII collagen
present in the sublamina densa region; hence, indirect
immunofluorescence on salt-split skin shows a “dermal”
staining pattern.23 However, in more than half of the patients
with epidermolysis bullosa acquisita, circulating antibodies
cannot be detected by indirect immunofluorescence.24 In the
current study, circulating antibodies could not be detected
by indirect immunofluorescence in 37.5% of epidermolysis
bullosa acquisita (three out of eight). In such cases, detection
of antibodies against Type VII collagen by ELISA may be
employed, but the sensitivity of ELISA too is only 23% in
the indirect immunofluorescence negative subset. Thus, a “u”
serration pattern on direct immunofluorescence microscopy
may be considered the gold standard for the diagnosis of
epidermolysis bullosa acqusita.10 Buijsrogge et al. observed
that serration pattern analysis by direct immunofluorescence
microscopy identified almost three times more epidermolysis
bullosa acquisita patients who would have most likely been undetected because of their clinical resemblance to other
pemphigoid diseases, together with negative serology
Conclusion
To conclude, all clinicians should make every attempt to classify subepidermal autoimmune blistering disorders as it has therapeutic and prognostic implications. A definite diagnosis could be offered to a majority of the patients in the present study using a combination of indirect immunofluorescence and serration pattern analysis so that appropriate clinical management could be instituted. Serration pattern analysis can be done using the same biopsy specimens at no extra cost either to the patient or laboratory. The technique is also fairly simple and can be mastered with ease. Therefore, inclusion of serration pattern analysis in the diagnostic armamentarium of subepidermal autoimmune blistering disorders is strongly recommended.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the
study.
Financial support and sponsorship
This study was partially supported by IADVL through
"IADVL postgraduate thesis grant" (2017-18) which was
awarded to Dr Sukriti Arora.
Authors also thank Dr SL Sujitha Reddy , junior resident,
department of Dermatology, Kasturba Medical College,
Manipal for helping in the schematic diagram.
Conflicts of interest
There are no conflicts of interest
References
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet 2013;381:32032.
- Kasperkiewicz M, Zillikens D, Schmidt E. Pemphigoid diseases: Pathogenesis, diagnosis, and treatment. Autoimmunity 2012;45:55-70.
- Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: Clinical features, diagnosis, and management. Clin Rev Allergy Immunol 2018;54:26-51.
- Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: The European dermatology forum consensus in collaboration with the European academy of dermatology and venereology. Br J Dermatol 2015;172:867-77.
- Koga H, Prost-Squarcioni C, Iwata H, Jonkman MF, Ludwig RJ, Bieber K. Epidermolysis bullosa acquisita: The 2019 update. Front Med (Lausanne) 2019;5:362.
- Vodegel RM, Jonkman MF, Pas HH, de Jong MC. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other sub-epidermal bullous autoimmune diseases. Br J Dermatol 2004;151:112-8.
- Buijsrogge JJ, Diercks GF, Pas HH, Jonkman MF. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165:92–98.
- Terra JB, Pas HH, Hertl M, Dikkers FG, Kamminga N, Jonkman MF. Immunofluorescence serration pattern analysis as a diagnostic criterion in antilaminin-332 mucous membrane pemphigoid: Immunopathological findings and clinical experience in 10 Dutch patients. Br J Dermatol 2011;165:815-22.
- Terra JB, Meijer JM, Jonkman MF, Diercks GF. The n-vs. u-serration is a learnable criterion to differentiate pemphigoid from epidermolysis bullosa acquisita in direct immunofluorescence serration pattern analysis. Br J Dermatol 2013;169:100-5.
- Terra JB, Jonkman MF, Diercks GF, Pas HH. Low sensitivity of TypeVII collagen enzyme-linked immunosorbent assay in epidermolysis bullosa acquisita: Serration pattern analysis on skin biopsy is required for diagnosis. Br J Dermatol 2013;169:164-7
- Meijer JM, Atefi I, Diercks GF, Vorobyev A, Zuiderveen J, Meijer HJ, et al. Serration pattern analysis for differentiating epidermolysis bullosa acquisita from other pemphigoid diseases. J Am Acad Dermatol 2018;78:754-9.
- Rao R, Bhogal B, Groves R. Antigen identification using skin deficient in basement-membrane protein: A novel tool for the diagnosis of subepidermal immunobullous diseases. Clin Exp Dermatol 2013;38:289-94.
- Gammon WR, Briggaman RA, Inman AO III, Queen LL, Wheeler CE. Differentiating anti-lamina lucida and anti-sublamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chlorideseparated skin. J Invest Dermatol 1984;82:139-44
- Ghohestani RF, Nicolas JF, Rousselle P, Claudy AL. Diagnostic value of indirect immunofluorescence on sodium chloride-split skin in differential diagnosis of sub-epidermal autoimmune bullous dermatoses. Arch Dermatol 1997;133:1102-7
- Meijer JM, Diercks GF, Schmidt E, Pas HH, Jonkman MF. Laboratory diagnosis and clinical profile of anti-p200 pemphigoid. JAMA Dermatol 2016;152:897‐904.
- Prost-Squarcioni C, Caux F, Schmidt E, Jonkman MF, Vassileva S, Kim SC, et al. International bullous diseases group: Consensus on diagnostic criteria for epidermolysis bullosa acquisita. Br J Dermatol 2018;179:30-41.
- Carey B, Setterfield J. Mucous membrane pemphigoid and oral blistering diseases. Clin Exp Dermatol 2019;44:732-9.
- Setterfield J, Shirlaw PJ, Kerr-Muir M, Neill S, Bhogal BS, Morgan P, et al. Mucous membrane pemphigoid: A dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br J Dermatol 1998;138:602-10.
- Lau I, Goletz S, Holtsche MM, Zillikens D, Fechner K, Schmidt E. Anti-p200 pemphigoid is the most common pemphigoid disease with serum antibodies against the dermal side by indirect immunofluorescence microscopy on human salt-split skin. J Am Acad Dermatol 2019;81:1195‐7.
- Goletz S, Zillikens D, Schmidt E. Anti-p200pemphigoid. J Am Acad Dermatol 2014;71:185-91.
- Dilling A, Rose C, Hashimoto T, Zillikens D, Shimanovich I. Anti-p200 pemphigoid: A novel autoimmune sub-epidermal blistering disease. J Dermatol 2007;34:1-8.
- Dainichi T, Koga H, Tsuji T, Ishii N, Ohyama B, Ueda A, et al. From anti-p200 pemphigoid to anti-laminin γ1 pemphigoid. J Dermatol 2010;37:231-8.
- Woodley DT, Briggaman RA, O’Keefe EJ Inman AO, Queen LL, Gammon WR. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med 1984;310:1007-13.
- Gammon WR, Kowalewski C, Chorzelski TP, Kumar V, Briggaman RA, Beutner EH. Direct immunofluorescence studies of sodium chlorideseparated skin in the differential diagnosis of bullous pemphigoid and

